OncoSec declares Publication In Nature Gene medication Demonstrating Efficacy Of IL-twelve Intratumoral Gene Electrotransfer
OncoSec's lead programme, ImmunoPulse IL-twelve, is currently in clinical Growth for metastatic skin cancer & triple-negative breast Cancer disease. Cautionary Note Regarding Forward-Looking StatementsThis press release contains "forward-looking statements" within the meaning of the U.S. Forward-looking statements could be identified with words like "could," "probably," "going to," "propose," "look forward to," "possibility," "understand," & similar references to aftertime periods. Forward-looking statements are neither historical realities nor assurances of aftertime performance. Undue reliance ought not be placed on forward-looking statements, that speak just as of the date they are made.
Parkinson's Patients' Long-term Movement gets better by Voyager's Gene medication
As it stated in A single Organization of Voyager Therapeutics gene medication gets better features Parkinson's patients' movement up to 3 years later, a Phase 1b experience indicates. The enzyme is responsible for converting the curing levodopa into dopamine, the signaling molecule which is missing in Parkinson's patients. The neuron dyinges protect the substantia nigra from being enable to of transform oral levodopa to dopamine. It too allowed patients to make substantial reductions in their Utilize of daily oral levodopa & other Parkinson's medications. VY-AADC01 too generated durable improvements in other measurements of motor function, including reductions in daily on-time by troublesome dyskinesia, or Compelling muscle movements.Gene medication for Age-linked Macular Degeneration - Pipeline test | Technavio
Technavio has published a Fresh market study report on gene medication for age-linked macular degeneration from 2018-2022. (Graphic: Business Wire)Technavio has published a Fresh market study report on gene medication for age-linked macular degeneration from 2018-2022. (Graphic: Business Wire)LONDON--(BUSINESS WIRE)--Technavio has reported its market study report on gene medication for age-linked macular degeneration. Gene medication for age-linked macular degeneration - market overviewMacular degeneration is a condition in that, macula, a portion of the retina, gets damaged or deteriorated. This condition usually affects individuals who are aged 50 years & above & therefore, it is called age-linked macular degeneration (AMD).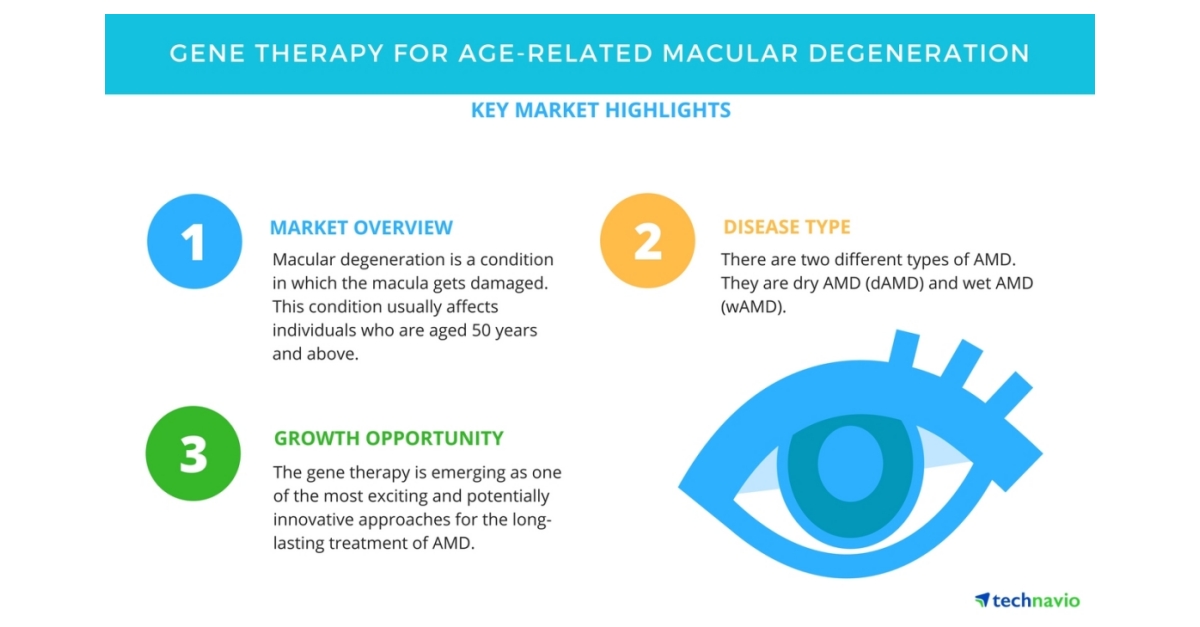
collected by :Lucy William



No comments:
Post a Comment