as declared in hartfordcitynewstimes
Zika Vaccine Trials Begin In Baltimore
Zika Vaccine Trials Begin In BaltimoreTodaySome clouds this morning will give way to generally sunny skies for the afternoon.Winds light and variable.TonightClear to partly cloudy.
besides cbslocal
Zika Vaccine Being Tested In 40 People « CBS Miami
Zika Vaccine Being Tested In 40 People « CBS Miami
Follow CBSMIAMI.COM: Facebook | TwitterMIAMI (CBSMiami) – A Philadelphia based-lab is working on developing a Zika vaccine, and they say the early results seem promising."Late fall last year we were watching like everybody else, the epidemic in Brazil and South America," said Inovio Pharmaceuticals President Dr. Joseph Kim.Kim teamed up with Dr. David Weiner at Philadelphia's Wistar Institute to create a safe vaccine to battle the Zika virus.
by the same token on wsj
A Zika Vaccine Poses a Number of Problems
A Zika Vaccine Poses a Number of ProblemsDr. W. Ian Lipkin, an epidemiologist, seems to be unaware of the many obstacles to the development of vaccines to prevent Zika ("The Coming Trials of Generation Zika," op-ed, Sept. 7).Increasingly defensive about accusations that drugs and vaccines are inadequately tested for safety, the safety-obsessed FDA regulators in recent years have required massive, hugely expensive and time-consuming clinical trials designed to detect even very rare side effects, especially for vaccines administered to healthy subjects, as would be the...
furthermore seekingalpha
Inovio - First To Zika And MERS Vaccine Trials, Has 4 Near-Term Catalysts - Inovio Pharmaceuticals, Inc. (NYSEMKT:INO)
Inovio - First To Zika And MERS Vaccine Trials, Has 4 Near-Term Catalysts - Inovio Pharmaceuticals, Inc. (NYSEMKT:INO)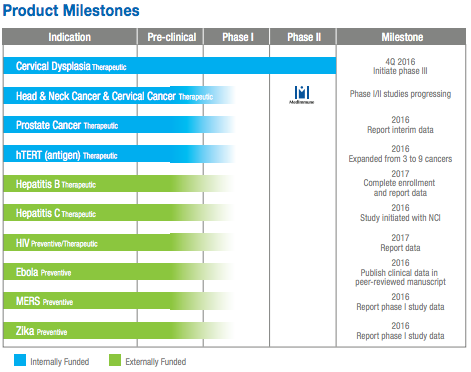
By year-end, Inovio Pharmaceuticals (NYSEMKT:INO) expects to launch or report on four clinical trials for its DNA vaccines and therapies; any or all could be near-term stock catalysts, if successful - or stock deflators, if not.Here they are, with potential revenue scenarios:Drug Candidate Potential Catalyst by Year-End If FDA/EMA-Approved, Potential Revenue Scenario VGX-3100: Cervical dysplasia therapy Initiate Phase III trial $520 million/year INO-5150: Prostate cancer therapy Report interim Phase I data $35 million/year (royalties only), plus any development milestone payments Zika vaccine Obtain data from first Phase I trial $80 million, one-time (royalties only) MERS vaccine Report Phase I data $210 million, one-time Click to enlargeThe VGX-3100 drug candidate has by far the greatest potential value to Inovio.The revenue potentials for the other three candidates pale in comparison, due to royalty-only revenues, one-time administration to a fixed population of patients, or both.



No comments:
Post a Comment